Patricia S. Steeg, chief of the Center for Cancer Research at the National Cancer Institute, argues in Nature [2012, 485, S58a] that most people with breast cancer die as a result of metastases (spread). Clinical trials, however, are only designed to evaluate a drug’s ability to shrink established tumors rather than its ability to block metastatic processes. Because there is more to cancer than tumor growth, those who are at a high risk of recurrence or have limited metastatic disease may gain greater benefit from drugs that keep cancer cells from entering the bloodstream, surviving in circulation, affecting the immune system, or invading other tissues and distant organs than they would from drugs focused only on tumor shrinkage. Unless clinical trials are redesigned to study metastasis-preventive compounds, Steeg says, we are not likely to see a decline in breast cancer metastasis.
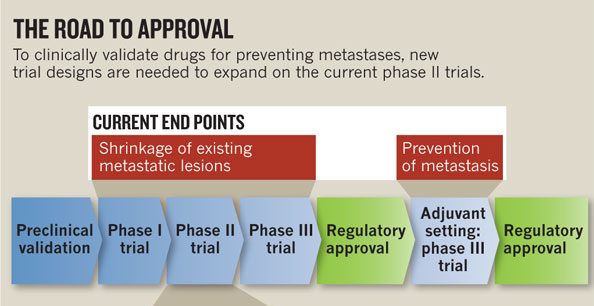
Steeg explains the regulatory approval process. Once a potential drug has been validated in the preclinical setting, it must move through three phases of clinical trials:
- Phase I trial: The drug is tested for safety and toxicity in the hardest-to-treat patients: those with metastatic cancer that is resistant to treatment.
- Phase II trial: If the drug is adequately safe, it is then evaluated in terms of its ability to shrink established tumors.
- Phase III trial: If the drug passes Phases 1 and 2, it must then demonstrate a clear benefit to patients over the current standard of care.
If a drug passes through the first three phases, additional trials could be conducted in the adjuvant setting (in combination with the standard treatment regimen) to find out if the drug also has the ability to prevent metastases. Although there are examples of drugs that followed this route (i.e., Tamoxifen, Herceptin, and cytotoxic drugs), these kinds of studies are expensive and require large numbers of patients compared to earlier phase trials. Thus, they are rare.
For Steeg, the clinical-trial design is a major barrier to the development of drugs that could prevent breast cancer metastasis. Tested in the current system, such drugs would fail because tumor shrinkage –instead of the time until a new metastasis occurs– is the measured end point. “The oncology community as a whole needs to commit to doing something different,” says Steeg. She proposes a detour from the currently accepted road to approval, using a new clinical-trial design that is appropriate for metastasis prevention. Conducting randomized phase II trials for metastasis prevention would also apply to other cancers besides breast cancer.
RESEARCH BRIEFS
BCC highlights scientific research studies that bridge social science, culture, and medicine as it relates to breast cancer. In addition to reporting on methods or key findings, research briefs highlight the implications of the research. We encourage synopses of research published within the last 6-12 months. However, classic studies that speak to current issues are also considered. Research briefs are approximately 350 words.
The post Research Brief — “The Right Trials” appeared first on Breast Cancer Consortium.